- Shen, H. and Laird, P. W.: In Epigenetic Therapy, Less is More. Cell Stem Cell, 10(4):353-354, 2012; “This elegant study makes the most convincing case to date that these DNA methyltransferase inhibitors act to reshape the epigenetic landscape of self-renewing clonogenic cells, rather than act as traditional cytotoxic agents, at lower doses. ……...Epigenetic drugs may become a standard measure in clinical treatment of a variety of cancers.”
- Science Daily: Scientists Reprogram Cancer Cells With Low Doses of Epigenetic Drugs, Mar 22, 2012. (ScienceDaily is an award-winning science news website since 1995)
- Science News: Old cancer drugs offer new tricks, April 2, 2012. (Science News, since 1922, is an award-winning biweekly news magazine covers important and emerging research in all fields of science.)
- Oncology & Biotech News: Epigenetics Breathes New Life Into Older Cancer Drugs, June 22, 2012.
- E3: Dosage Makes All the Difference! Zahnow & Baylin Labs Show Epigenetic Reprogramming of Cancer, April 3, 2012.
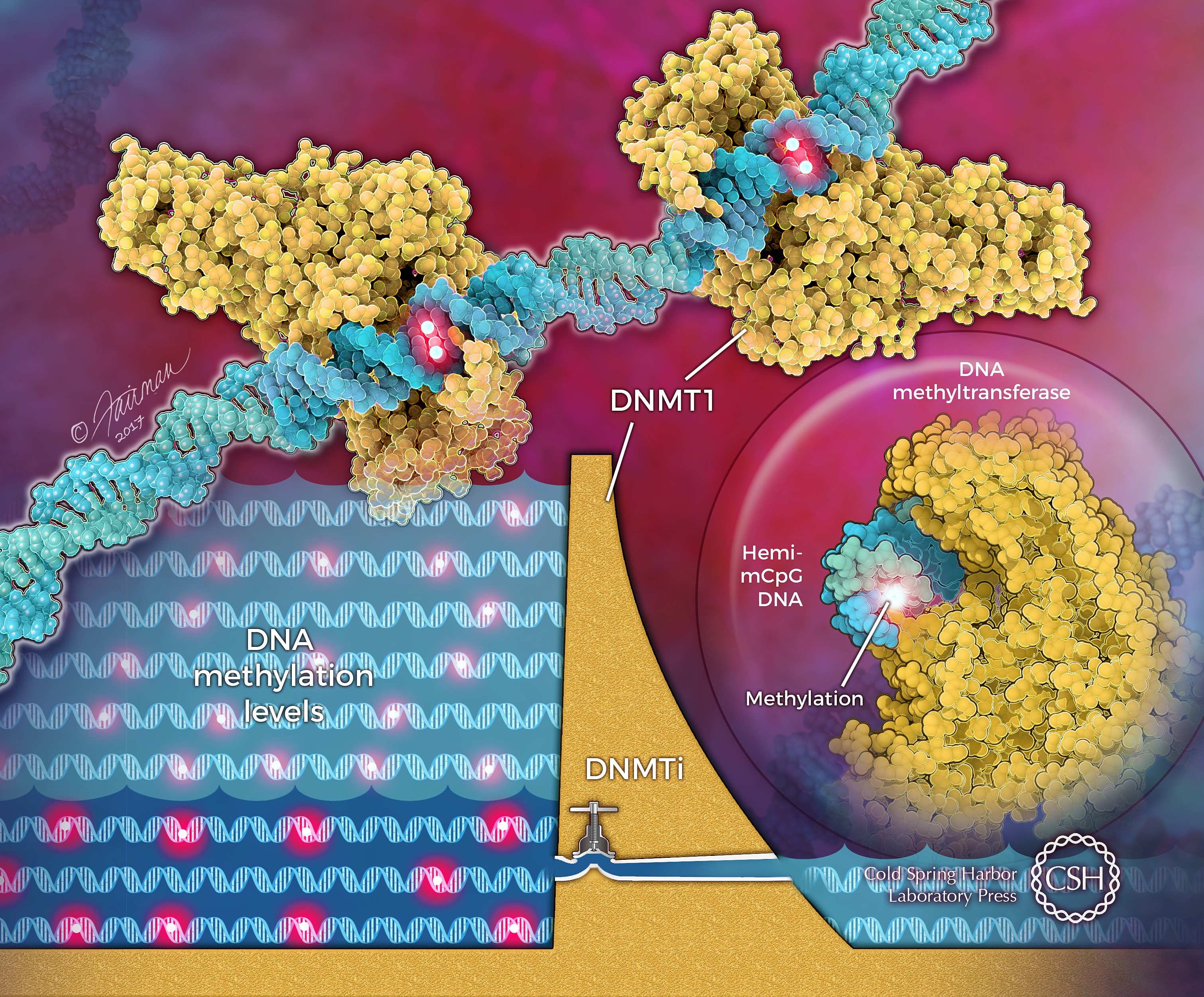
Epigenomic profiling revealed critical threshold levels of DNA methyltransferase (DNMT) 1 to maintain DNA methylation across the genome in human cancer cells.
There has been a controversy over whether DNMT1 is the sole DNMT in maintaining cancer-specific DNA methylation. Previous research efforts from world-renown epigenetic labs through knockout or knockdown studies yielded conflicting results. Besides, the studies were limited to certain genomic regions lacking the comprehensive insights of genome-wide epigenomic profiles. We used graded time-dependent shRNA approaches to create a DNMT1 gradient system, which contains clones derived from colorectal cancer cells, HCT116, with step-wise reduction of DNMT1 proteins. We discovered that lowering DNMT1 below a threshold level is required for maximal loss of DNA methylation at all genomic regions, including gene body and enhancer regions, and for maximally reversing abnormal promoter DNA hypermethylation and associated gene silencing to reexpress key genes. The study has significant clinical implications as it is difficult to reach this threshold with patient-tolerable doses of current DNMT inhibitors (DNMTIs). We showed that new approaches, like decreasing the DNMT targeting protein, UHRF1, can augment the DNA demethylation capacities of existing DNA methylation inhibitors for fully realizing their therapeutic potential.